Philips shows ‘substantial’ shortcomings in sleep apnea recall
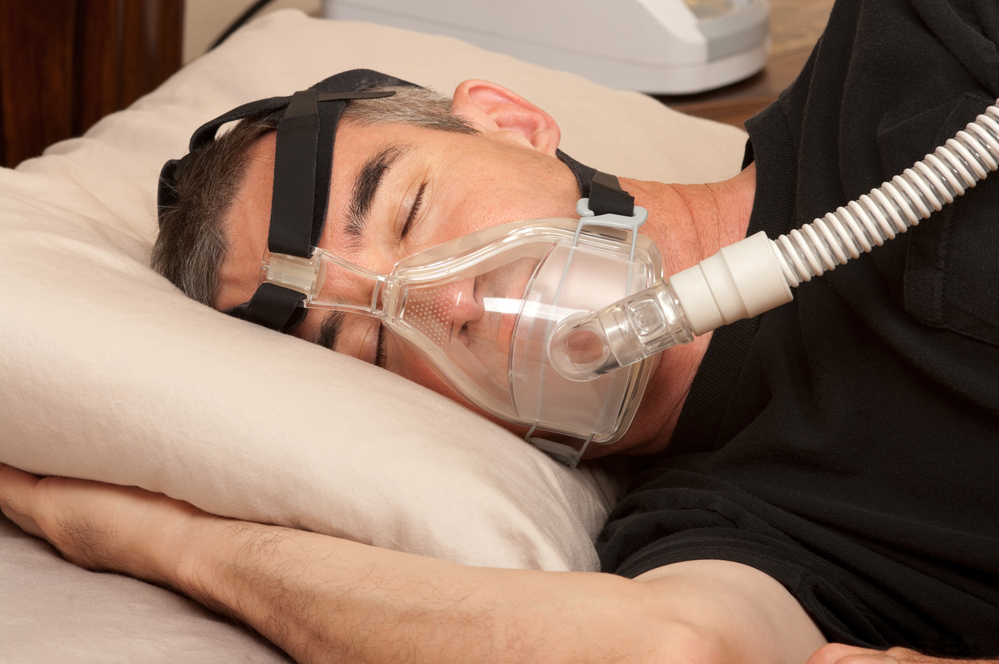
Medical technology group Philips has shown ‘substantial and structural’ shortcomings in its recall campaign for faulty sleep apnea technology, Dutch health service inspectors have told television current affairs show Nieuwsuur.
In particular, patients, doctors and others involved in the treatment of sleep apnea should have been better informed about the recall and the health risks posed by using the apparatus, the inspectors say.
In March this year, the FDA in America criticized the ‘inadequate’ provision of information and said most patients still have no idea when their equipment, which ensures they continue to breath at night, will be replaced.
Philips also told Nieuwsuur that 70% of Dutch patients are still using the old machines.
The company has revised its deadline for completing the recall several times, and now says 90% of patients should have been helped by the end of this year. In total, 5.5 million machines need to replaced or repaired.
Philips medical director Jan Kimpen told the programme that he understood patients’ unease and that the complexity and size of the recall operation had led to the problems with production and communication.
Philips said in June last year it had determined that there ‘are possible risks’ centred on foam used in the equipment to dampen noise. This, the company said, ‘may degrade into particles which may enter the device’s air pathway and be ingested or inhaled by the user’.
The foam may also leach certain certain chemicals as a gas and its degradation may be made worse by unapproved cleaning methods, Philips said.
Health impact
In the Netherlands, where some 50,000 people have the devices, lung specialists and the health inspectorate have recommended people continue to use them.
The FDA said last week it had received 21,000 health complaints from users, ranging from cancer to head aches and chest pain. It also reported 121 deaths although no link has been made to the apparatus.
Philips is due to publish its research on the health implications next month.
Monique Klaaver, a lung specialists, told Nieuwsuur that Philips’ communication with doctors and patients is ‘deplorable’. ‘We are talking about an essential medical aid here, not a vacuum cleaner,’ she said.
Thank you for donating to DutchNews.nl.
We could not provide the Dutch News service, and keep it free of charge, without the generous support of our readers. Your donations allow us to report on issues you tell us matter, and provide you with a summary of the most important Dutch news each day.
Make a donation