Philips to upgrade millions of breathing machines over foam fears
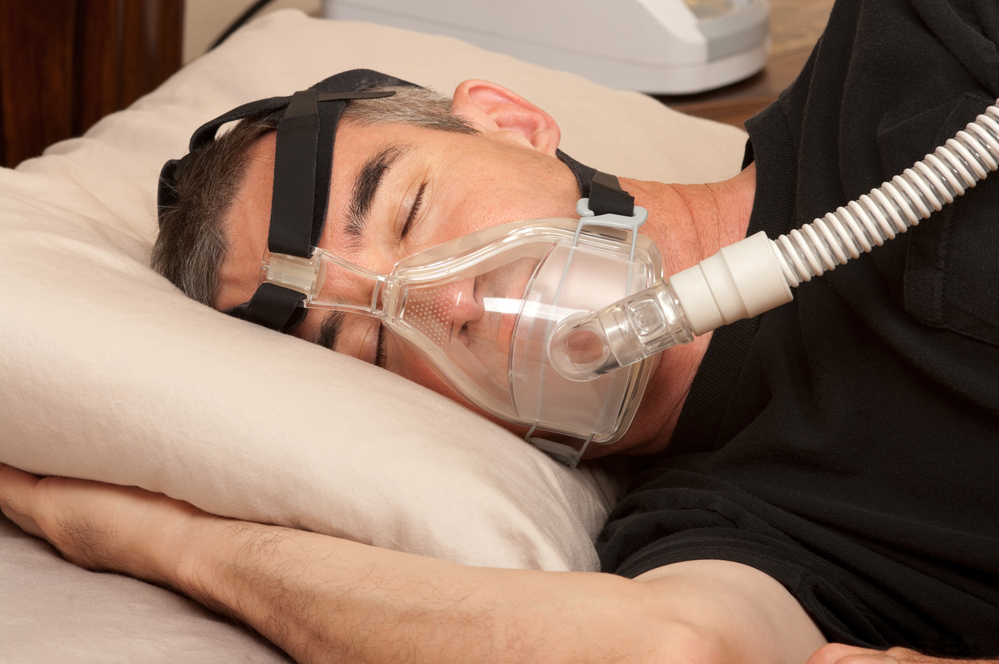
Philips has issued a recall notice for between three million and four million sleep and respiratory care devices because of possible health risks attached to foam used in the equipment to dampen noise.
Philips said that it had carried out tests and determined that there ‘are possible risks’ centred on the foam which ‘may degrade into particles which may enter the device’s air pathway and be ingested or inhaled by the user’.
The foam may also leach certain certain chemicals as a gas and its degradation may be made worse by unapproved cleaning methods, Philips said.
Philips said it has received reports of possible problems for patients involving foam degradation but none relating to possible chemical emissions. The potential risks of exposure to foam crumbs include headache, irritation, inflammation, respiratory issues, and possible toxic and carcinogenic effects.
Most of the problems have been found in Philips’ first generation Dreamstation devices used by people who suffer from sleep apnea.
The recall notification applies to the US only, where most of the devices were sold. For the rest of the world, Philips said, the statement should be seen as a ‘field safety notice’. Philips has not responded to DutchNews.nl’s request for clarification about the difference.
The company has said it is currently notifying regulatory agencies in the regions and countries where affected products are available and that it will replace the current sound abatement foam with a new material that is not affected.
In the meantime, people should stop using the devices and consult their doctor, Philips said.
Uncertainty
In the Netherlands, where some 50,000 people use the devices, doctors are calling on Philips to provide more information about the risks.
‘There is still a lot of uncertainty and this has been a huge surprise for us as a profession,’ lung specialist Monique Klaaver told broadcaster RTL Nieuws. She hopes Philips will soon provide more clarity and come up with an action plan.
Philips, she says, has not yet shared information about the health implications with doctors. ‘So we can’t really assess the risks,’ she said.
Philips has set aside €500m in total to fund the recall, but said it does not expect an impact on 2021 earnings.
Thank you for donating to DutchNews.nl.
We could not provide the Dutch News service, and keep it free of charge, without the generous support of our readers. Your donations allow us to report on issues you tell us matter, and provide you with a summary of the most important Dutch news each day.
Make a donation